
Passionate Researcher in the world of Acoustofluidics
Welcome to my Homepage!
🧑🏽🔬 I am an enthusiastic scientist working in the field of Acoustofluidics, i.e. particle manipulation using acoustic forces in microfluidic devices. In my project, I am developing solutions to extract Extracellular Vesicles from micro liter (0.004 mL) blood samples to detect new biomarkers for early disease detection and personalised medicine. https://www.acousome.com/
🌍My mission is to improve medical tools and find affordable solutions for personalised medicine to compete against currently uncurable disease and zoonotics. Further, I am interested in spreading science to the world and educate students as a group leader.
My Path
Abitur
High school diploma
Annette-von-Droste-Hülshoff-Gymnasium
2001-2010
Bachelor
Electrical Engineering
University of Wuppertal
2010-2013
Master
Electrical Engineering
Technical University of Munich
2013-2015
Ph.D.
Mechanical Engineering
ETH Zurich
Mechanics and Experimental Dynamics
Prof. Jürg Dual
2016-2021
Postdoctoral Researcher
Chemistry
ETH Zurich
Biochemical Engineering Laboratory
Prof. Paolo Arosio
2021-2023
Postdoctoral Researcher
Biomedical Engineering
Lund University
Acoustofluidics Group
Prof. Thomas Laurell
2023-current
Research Projects
Isolation of Extracellular vesicles from whole blood
European Innovaton Council (EIC) transition project (Acousome)
Link to layman explanation
Extracellular vesicles (EVs) are nanoscale messengers carrying molecular information that can serve as powerful biomarkers for early disease detection, but their small size makes isolation from blood highly challenging. To address this, we developed an acoustofluidic chromatography platform that isolates EVs directly from microliter plasma samples using a bead-packed microfluidic capillary actuated by ultrasound. The device achieves high-throughput enrichment with recoveries up to 42.9%, minimal protein contamination, and successful isolation of EV-sized particles for downstream analysis, offering a fast and efficient route toward diagnostic and therapeutic applications.

Intra-droplet single cell manipulation
Collaboration with the Bioanalytics Group at ETH headed by Prof. Dittrich
Link to layman explanation
Mass spectrometry (MS) analysis of the cell secretome could help identify promising candidates for directed evolution, but it requires separating the cell from its secretions to keep it alive. In this project, we present a droplet microfluidic method that uses acoustic forces to non-invasively manipulate and separate particles or cells from the surrounding supernatant within nanoliter droplets. The approach achieves up to 100% separation efficiency at 114 droplets/min for beads and yeast cells, and is versatile enough to handle mammalian cells and bacteria, providing a robust strategy for single-cell secretome sampling.
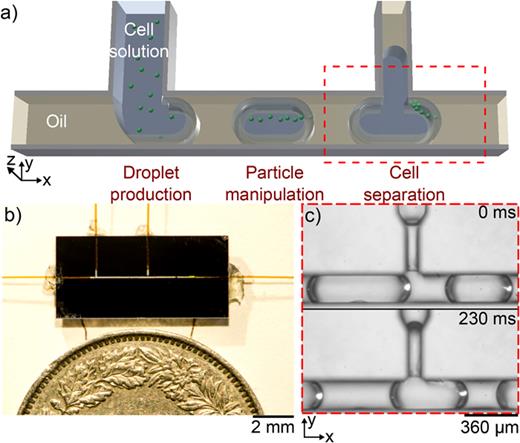
Medium exchange of bacteria for automated reprograming
Collaboration with the Bioprocess Laboratory at ETH headed by Prof. Panke
Link to layman explanation
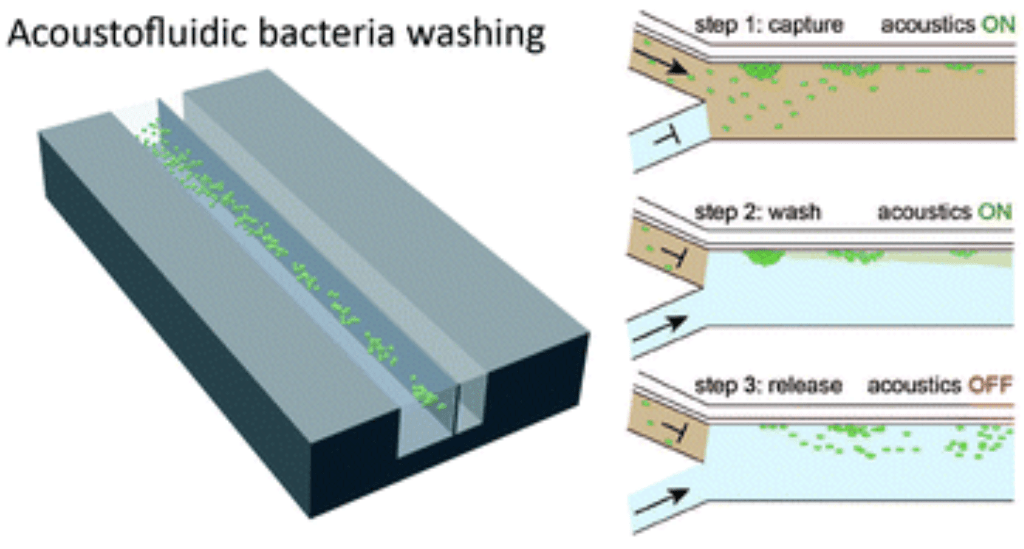
Genetically modified bacteria are powerful tools for producing tailored proteins, but reprogramming often requires cumbersome medium exchange steps. We developed an acoustofluidic device that enables non-invasive trapping and efficient medium exchange of E. coli, overcoming challenges posed by acoustic streaming. Operating at 10 μL/min, the device achieves 47 ± 3% cell recovery, while maintaining high electrocompetence with transformation efficiencies of 8 × 10⁵ CFU/μg DNA. This low-volume alternative to centrifugation simplifies and miniaturises a critical step in synthetic biology, advancing automation of microbiological and molecular engineering workflows.
Metal particle focusing for improved 3D-printing
Collaboration with the Laboratory of Thermodynamics in Emerging Technologies at ETH headed by Prof. Poulikakos
Link to layman explanation
In this project, we set out to improve the resolution of metal 3D printing by Direct Metal Laser Sintering, where the main limitations arise from the laser focus spot size and the metal powder particle dimensions. To address this, we developed an acoustofluidic approach that enables two-dimensional focusing of metal microparticles in acoustically excited round glass capillaries, offering a low-cost alternative to cleanroom-fabricated devices. Using bulk acoustic waves, we achieved robust focusing of 1 µm copper particles into a narrow line (60.8 ± 7.0 µm width, 45.2 ± 9.3 µm height) with a 90-fold local concentration increase, and even manipulated 1 µm polystyrene particles typically hindered by acoustic streaming. Numerical analysis revealed that unique streaming transitions lower the critical particle radius compared to rectangular channels, enabling applications such as particle ejection through 25 µm openings for processes that usually suffer from clogging, abrasion, or limited resolution.

Improved Silicon etching for special applications
Collaboration with the Multi-Scale Robotics Lab at ETH headed by Prof. Nelson
Link to layman explanation
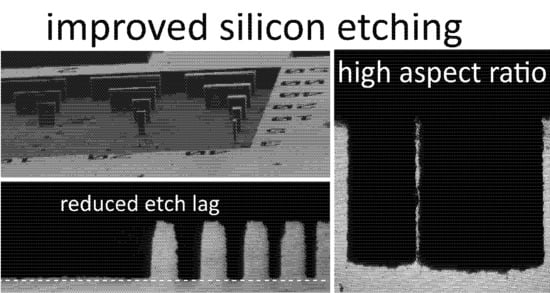
Deep reactive ion etching (DRIE) using the Bosch process enables high-aspect-ratio silicon microstructures for MEMS and microfluidics but is limited by etch lag and complex mask requirements. We present an optimized two-step Bosch process that reduces etch lag to below 1.5%, significantly improving etch uniformity. In addition, we demonstrate a three-step process capable of producing stable 6 μm wide structures with depths up to 180 μm, advancing miniaturization for biomedical applications.
Effect of shear and interfaces on protein aggregation
Collaboration with Janssen/Cilag in Schaffhausen
Link to layman explanation
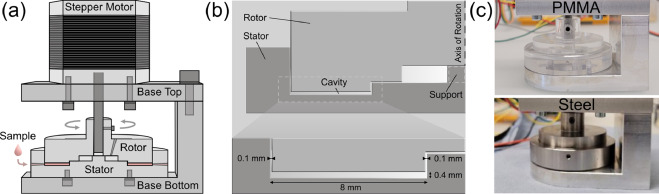
Protein aggregation caused by shear and interfacial stress is a major challenge in therapeutic protein and antibody production. We developed a microfluidic device that exposes small protein volumes to uniform shear, enabling us to separate flow effects from interfacial contributions by comparing PMMA and stainless-steel devices. Our results show that interfaces strongly mediate aggregation, with steel producing 3.5-fold fewer particles than PMMA, while surfactants prevent aggregation entirely—highlighting the device as a valuable tool for formulation stability testing in biopharmaceutical development.
My life as a Postdoc
Blog of my life as a Postdoc (arriving soon)
Acoustofluidics Wiki
This is a small summary of my Acoustofluidics expertise